Abstract
Introduction:
Chemoimmunotherapy with R-CHOP is the standard of care for newly diagnosed diffuse large B cell lymphoma (DLBCL) leading to high cure rates although treatment-related toxicities are significant among older adults. Reduced dose R-CHOP has been proposed to balance efficacy and safety, but there is considerable ambiguity in "whom" and "by how much" with the best available data on those >80y [Peyrade et al 2011]. In this study, we sought to document the variability in chemotherapy dosing among unselected older adults with DLBCL treated with R-CHOP at a large, urban academic center in the Deep South. In addition, we sought to determine how baseline clinical features impacted tolerance of treatment.
Methods:
We conducted a single-institution retrospective review of all adults with newly diagnosed DLBCL initiating chemoimmunotherapy, predominantly R-CHOP, between 1/2015 and 2/2021 at the University of Alabama at Birmingham. For the current analysis, the cohort was limited to patients >70y. We extracted information on baseline clinico-demographic variables (age, sex, race, albumin, ECOG performance status [ECOG PS], Charlson comorbidity index [CCI], cancer type, stage, Revised International Prognostic Index score) and treatment intensity (type and dose of chemotherapy at 1 st cycle). Dose reduction was defined as any pre-planned reduction in cyclophosphamide, vincristine, or doxorubicin from standard CHOP dosing for cycle 1 of treatment. The primary outcome of interest was ≥grade 3 treatment-related toxicity (using CTCAE v5.0) occurring up to 30 days from the last dose of R-CHOP; secondary outcomes included dose modifications (dose delays >7d, dose reductions and treatment discontinuation) and health care utilization (unplanned emergency department visits and hospitalizations). Given high missingness for ECOG PS, we performed multiple imputation with chained equations producing 10 imputed datasets. Finally, we built a multivariate logistic regression model to identify baseline characteristics associated with severe toxicity; putative risk factors included age, sex, ECOG PS, CCI, albumin and treatment intensity. We pooled regression coefficients across imputed datasets using Rubin's rules. All hypothesis testing were two-sided, and the level of significance was chosen as 0.05.
Results:
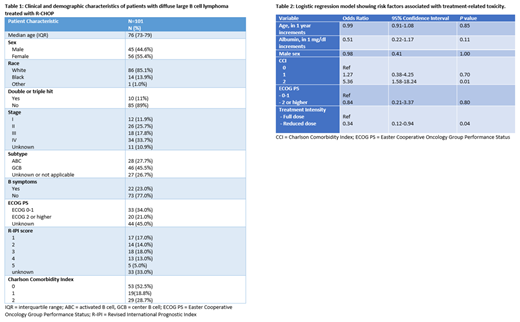
Of 287 patients with DLBCL initiating R-CHOP during the study period, 101 (35%) patients were included in our cohort. The median age was 76y (IQR 73-79), with 55% males and 85% whites. Stage IV disease was present in 33% of patients, and 11% had double or triple-hit subtype. Of those with non-missing ECOG PS (55%), 38% had ECOG PS ≥2, and 29% had a CCI ≥2 [Table 1].
Overall, 55% of patients received planned reduced dose treatment. Interestingly, 51% of patients <80y received reduced dose R-CHOP, whereas 32% of patients ≥80y still received full dose R-CHOP. Over half of patients (54%) experienced ≥grade 3 toxicity (42% hematologic toxicity, 27% non-hematologic toxicity). Dose delays (12%), reductions (12%), or discontinuations (17%) were frequent, and over a third (35%) of patients had unplanned hospitalizations during treatment. In a multivariate logistic regression model, reduced dose R-CHOP (Odds Ratio, OR 0.34; 95% CI 0.12-0.94; p value 0.038) was associated with decreased risk of ≥grade 3 toxicity, and CCI ≥2 (OR 5.35; 95% CI 1.58-18.25; p value 0.007) was associated with increased risk of ≥grade 3 toxicity. Age, albumin, and ECOG PS were not associated with risk of toxicity [Table 2]. Overall, only 11% of the observed inter-individual variability in toxicity could be explained by the above potential risk factors.
Conclusions:
Among older adults >70y with DLBCL, planned dose reductions based on anticipated tolerability were frequent, yet over half of patients experienced severe treatment-related toxicity. Treatment intensity and comorbidity burden were significantly associated with increased risk of toxicity, but age, albumin, and performance status were not. Overall, pre-treatment factors only explained about 11% of the observed variability in toxicity suggesting that a significant proportion of inter-individual variability in severe toxicity is unexplained by traditional evaluation. Future studies should examine the role of geriatric assessment and altered body composition in older adults to identify those at increased risk of severe toxicity from chemoimmunotherapy.
Narkhede: Genentech/Roche: Research Funding; Genmab: Other: Medical writing support, Research Funding; TG Therapeautics: Research Funding; Gilead: Research Funding. Mehta: Affirmed; Kite/Gilead; Roche-Genetech; Celgene/BMS; Oncotartis; Innate Pharmaceuticals; Seattle Genetics; Incyte; Takeda; Fortyseven Inc/Gilead; TG Therapeutics; Merck; Juno Pharmaceuticals/Bristol Myers Squibb: Research Funding; Seattle Genetics; Incyte; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics; Incyte; TG Therapeutics: Consultancy. Giri: PackHealth: Research Funding; CareVive: Honoraria, Research Funding.